Our services
Before building the perfect solution, we always start with a deep analysis of your needs...
GAMP Development
Our development approach is based upon ISPE GAMP to fulfill pharmaceutical regulatory requirements. GAMP is the acronym of Good Automated Manufacturing Practices, which has been published by the International Society for Pharmaceutical Engineering. Its purpose is to set the requirements for software development used in the pharma industry.Requirements
Define project goals and system functions and operations by analyzing end-user requirements and functional needs.
Design
Translate requirements and functional needs into a Design specifications, including screen layouts, business rules, process diagrams, use case...
Development
Build all required components, database structure and user interface to implement all use cases.
Maintenance
Continous evaluation of the system performances and system enhancement through change control process.
Verification
Create tests cases for all major development phases (unit, integration and deployment) to confirm that the system perform according to specifications
21CFR Part 11 Assessment / Gap Analysis
In March of 1997, FDA issued final part 11 regulations that provide criteria for acceptance by FDA, under certain circumstances, of electronic records, electronic signatures, and handwritten signatures executed to electronic records as equivalent to paper records and handwritten signatures executed on paper. These regulations, which apply to all FDA program areas, were intended to permit the widest possible use of electronic technology, compatible with FDA's responsibility to protect the public health.
After a while, concerns have been raised that some interpretations of the part 11 requirements would unnecessarily restrict the use of electronic technology in a manner that is inconsistent with FDA's stated intent in issuing the rule. The FDA then decided to narrow the scope of Part 11 to help reduce de cost of general compliance for computerized systems used in regulated environment and to protect what really needs to be protected.
In order to help you to comply with Part 11 requirements, we have developed a documented approach that will determine first if your computerized system qualifies to Part 11.
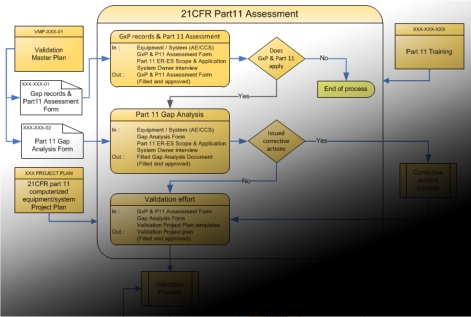
Here are the major steps found in our approach
- We generate a workflow analysis of your process
- We determine if your computerized system is taking part in generating and/or managing GxP records
- We determine if these GxP records and computerized system can be disqualified due to the paper based utilisation of the produced data (word processor effect)
- If GxP records are qualifying, we determine if Part 11 apply on produced records
If Part 11 applies, then we will perform these next steps
- Gap Analysis (to determine which requirement is not fulfill by your system )
- Corrective Actions (to repair or mitigate the item found in Gap Analysis)
- Validation Effort (to determine what tests need to be added to Qualification Protocol)
- Qualification of equipment
To be able to patch your system deficiencies, corrective actions can range from procedural mitigation to software development. The most notable deficiency found on analytical and production systems is illegal access to system data. This is mainly due to system limitation, which needs to be started with administrator's rights. This requirement sometimes, leaves the user to gain access to data directories which might potentially lead to data falsification or deletion.
To resolve this problem, we have created a patch, that enable all your users to connect to the system with their standard Windows user account, and start any software with administrator's rights, within current user's session...
You want to know more, ask us about our XRunAs application.
Business Process Automation and Optimization
We can develop custom solutions that will help your enterprises to automate its business processes. You will some time find off the shelf's tools that will resolve part of your problems; maybe you will use a spreadsheet to solve a situation where your other tools couldn't make it. If you find yourself in this situation, maybe it is time to think about process automation and optimization.
Our experience in this field showed us that a short term Return On Investment (ROI) has always been a valid proof of success. The end results of process automation will be; increasing of employee's efficiency and reducing of general costs. Our approach allows us to create highly expandable and modular systems.
6 steps to BPAO
- Identify the source of the pain
If you are thinking about BPA, it is probably because you already pin point areas in your work environment where time and money is lost. A simple meeting with your collegues and employees to know where optimization could be applied, will provide you valuable information about your company's health... - Document your process
Documenting your processes will help getting a better understanding of all interactions between departments, tasks and responsibilities and identify bottlenecks and current problems. - Process Analysis
To understand what you will gain from BPAO you must first, get the numbers. A time and costs analysis if primordial if you want to make a precise ROI calculation after project completion. - Plan for the new approach
Make sure you don't try to solve everything at the same time. From the results of previous analysis, select the sub process from which you would gain the biggest results. Start with only one big problem, solve the problems and gain respect from both upper management and your employees. The rest will be easy to implement. And by the way, don't forget to be creative... - Select your solution provider
Not all problems should be solved by customized solutions. In some case, you could find an Off-the-shelf software that will resolve your problem. If not, we are here to help... - Calculate your ROI
Before project ROI estimation and after project ROI calculation should be compared to determine whether the project was on target. This step is important to obtain upper management approval to persue BPA on other items found in analysis phase.
Call for Tenders Process Management
If you are planning to acquire an existing system on the market, and you don't want this process turns into a suppliers representation circus you need to call for tenders. Too often, companies make the mistake of meeting suppliers before even knowing their own needs. Under these conditions, most of the time, the company ends up with the best seller's system, instead of the one that would best meet their needs...
In order not to fall into this trap, we will help you set up a process to create and manage your call for tenders.
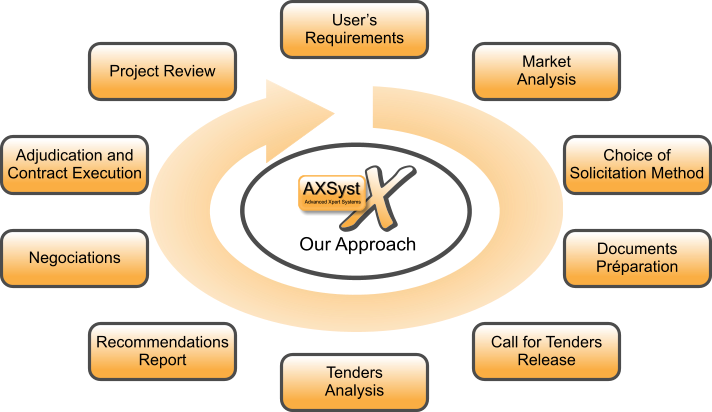
10 Steps to Create a Call for Tenders Process
- User’s Requirements
Functional needs, prioritization of needs and levels of services, organizational impact and business processes, budget estimate... - Market Analysis
Preliminary search to determine what is on the market and target identification for the launch of tenders... - Choice of Solicitation Method
Direct requests for proposals, public requests or on invitation, Electronic Tendering Platforms, Newspapers... - Documents Préparation
Creation of all documentation related to tender, the analysis process and the candidates selection criteria. - Call for Tenders Release
Launch of the call for tenders on the chosen medium and monitoring process. - Tenders Analysis
Impartial analysis of all received offers based on predetermined selection criteria. - Recommendations Report
Analysis report of all candidates with detailed results ranking. - Meetings and Negociations
Meetings with candidates who performed well in the analysis phase and negotiations on points that could be contentious. - Adjudication and Contract Execution
Choice of supplier, signings, startup management and work progress management until final delivery - Project Review
Feedback on the project, what whent good and wrong, lessons learned, return on system roll-out by the customer.
New concept development
You have a great idea but don't have the knowledge nor
the resources to bring it to life?
We can help you to make the last steps to market...
5 Steps to bring your software on the market
- Competition Analysis
Does your product already exist? Even though your idea is brilliant, There might be someone else out there that had the same though process and came to the same conclusion. It might not be the end of your dream, but it is imperative to know who are your competitors and understand their capabilities. - Market Validation
A good product is a product that sells... An early market study can help you save a lot of time and money. Before spending in the development of a new concept, you must validate ist market potential against true client's needs. - Business plan
This step is promordial if you want to obtain financing and mandatory to define your new concept's business stategies. - Create your application
Before developping a new system, it is imperative to take a brake and make a thorough analysis of the system's requirements and functionalities. A good analysis process make coding easier. - Promotional Campaign
To get visibility, you needs to select the right promotional channel to get in contact with the targeted audiance defined in your business plan.
Web applications
We develop Web applications compliant to HTML5 and CSS3 standards to fulfill your internal and external needs. Built on Apache, PHP, JavaScript and MySQL, our platform independent systems can be executed on any popular browsers.